Introduction: The pathophysiology of chronic graft-vs.-host disease (cGVHD) is complex, with many cell types playing critical roles. B cells are involved in cGVHD pathobiology, and strategies that eliminate or inhibit B cells (rituximab, ibrutinib) can be therapeutic in advanced disease. We previously demonstrated effective prevention of cGVHD in a phase II trial of B cell depletion with rituximab. Obinutuzumab (Obin, Genentech) is a 2 nd generation monoclonal anti-CD20 antibody with enhanced B cell depletion activity over rituximab. Here we present the results of a randomized trial of Obin for the prevention of cGVHD.
Methods: We performed a randomized double-blind, placebo-controlled trial of B cell depletion using 4 doses of Obin to determine if steroid-requiring cGVHD (SR-cGVHD) could be prevented 12 months from HSCT (NCT# 02867384). Eligible subjects previously underwent myeloablative or reduced intensity 7-8/8 HLA-matched, related or unrelated HSCT using peripheral blood stem cells (PBSC) and tacrolimus-based primary GVHD prophylaxis without T cell depletion. Subjects without evidence of cGVHD, active Gr II-IV acute GVHD, or malignancy were enrolled between 60-90 days following HSCT. Eligibility included adequate hematopoiesis (ANC > 1000/mL, plt > 50 000/mL) and stable donor chimerism (> 80%). All subjects provided informed consent. Obin (1000 mg iv) or placebo was administered at 3, 6, 9 and 12 months after HSCT (+/- 10 days) with acetaminophen and diphenhydramine prior to each study drug dose. The primary endpoint was the cumulative incidence (CI) of SR-cGVHD, which was felt to be a more objective measurement of cGVHD severity than NIH cGVHD stage alone.
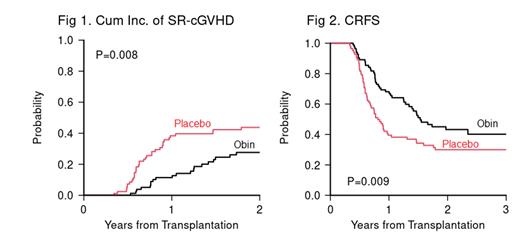
Results: 181 subjects were enrolled (92 Obin, 89 placebo). 167 subjects who have been followed for >1 year from HSCT (84 Obin, 83 placebo) are included in this analysis. Baseline clinical characteristics, including age, recipient/donor gender, donor type/match, primary malignancy/DRI, conditioning intensity and HCT-CI, and prior Gr II-IV GVHD were balanced. The median number of study drug doses was equivalent (median 2.5 vs. 2 for Obin and placebo groups). For the entire cohort, the median follow-up time among survivors was 24 months (range 4, 74). The primary endpoint, the CI of SR-cGVHD at 12 months from HSCT, was significantly reduced with Obin - 11% (95% CI 5.8-20) in comparison with placebo - 38% (95% CI 28-49) (p=0.008, Fig 1). The corresponding rates of NIH Moderate-Severe cGVHD at 12 months were 20% vs. 35% (p=0.069). The rate of SR-cGVHD by 2 years remained lower in the Obin group (28% vs. 44%). In multivariable modeling, only study arm (Obin vs. placebo) was associated with SR-cGVHD (p=0.01). Obin was safe and well tolerated with only 5 Gr 3-4 infusion reactions (placebo - 0). There was more Gr 3-4 neutropenia (32.1% vs. 4.8%) but only 1 case of neutropenic fever in the Obin group. Neutropenia responded to G-CSF, administered at investigator discretion. There were 9 non-relapse related deaths in the Obin arm (COVID 3, infection 2, pulmonary 2, acute GVHD 1, unknown 1) compared with 3 in the placebo arm (unknown 2, pulmonary 1) (p=NS). There was no difference in the CI of relapse at 2 years (20% vs. 28%, p=NS). The SR-cGVHD-free, relapse-free survival in the Obin group was superior (CRFS, 43% vs. 30%, p=0.009, Fig 2) without a change in overall survival 82% vs. 86%, p=NS). CD19 + B cell depletion was complete in the Obin arm through 1 year (median cell count 0) while B cell recovery was normal in the placebo arm (p<0.0001 at 3, 6, 9, 12 months) and remained lower at 24 and 36 months (36 month median 55.5 vs. 484.4 cells/mL, p=0.02). Similar findings were noted for CD19 +CD27 + memory B cells and CD19 +CD27 - naïve B cells. No differences in T cells were noted, but NK cells were reduced in the Obin arm at late time points. H-Y antibody studies in F to M donor/recipient pairs are underway.
Conclusions: In subjects undergoing well-matched PBSC HSCT using tacrolimus-based GVHD prophylaxis, a strategy of B cell depletion with 4 doses of Obin significantly reduces the requirement for corticosteroids for treatment of cGVHD and significantly increases CRFS. Obin is well tolerated and is associated only with transient neutropenia. Further studies may determine whether prolonged B cell depletion may be of greater utility, however this double-blind, placebo-controlled randomized trial supports practice changing cGVHD prophylaxis in tacrolimus-based HSCT.
OffLabel Disclosure:
Cutler:Pluristem Therapeutics: Other: DSMB; Allovir: Other: Data Safety Monitoring Board (DSMB); Sanofi: Consultancy; InhibRx: Consultancy; Ruth L. Kirschstein Postdoctoral Individual National Research Service Award: Research Funding; Oxford Immune Algorithmics: Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy; Astellas: Consultancy; Cimeio: Membership on an entity's Board of Directors or advisory committees. Ho:Orca Bio: Consultancy; Omeros: Consultancy; Allovir: Consultancy; Alexion, AstraZeneca Rare Disease: Consultancy; CareDx: Research Funding; Jazz Pharmaceuticals: Research Funding; Omeros: Research Funding; Alexion, AstraZeneca Rare Disease: Speakers Bureau. Koreth:Gentibio: Consultancy; Mallinckrodt: Membership on an entity's Board of Directors or advisory committees; Equillium: Consultancy; Cue Biopharma: Consultancy; Biolojic Design: Consultancy; Tr1x: Consultancy; Amgen: Research Funding; Clinigen Labs: Consultancy, Research Funding; BMS: Research Funding; Miltenyi Biotec: Research Funding; Regeneron: Research Funding; Cugene: Membership on an entity's Board of Directors or advisory committees; Equillium: Research Funding. Kelkar:CareDx: Research Funding. Defilipp:Incyte: Consultancy, Research Funding; Regimmune: Research Funding; Taiho Oncology: Research Funding; Sanofi: Consultancy; MorphoSys: Consultancy; Inhibrx: Consultancy; PharmaBiome AG: Consultancy; Ono Pharmaceutical: Consultancy. El-Jawahri:GSK: Consultancy; Incyte Corporation: Consultancy; Novartis: Consultancy. Couriel:SeaGen: Consultancy; Incyte: Consultancy. Lee:BMS: Honoraria; Fresenius Kabi: Consultancy; Kadmon: Honoraria; Sanofi: Consultancy, Honoraria; Kite Pharma: Honoraria, Speakers Bureau; Incyte Corp: Consultancy, Research Funding. Soiffer:Jasper: Consultancy; NMPD - Be the Match, USA: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy; Smart Immune: Consultancy; Neovii: Consultancy; Bluesphere Bio: Consultancy; Vor Bipharma: Consultancy; Juno Therapeutics/ BMS/Celgene USA: Other: Data Safety Monitoring Board. Miklos:Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Umoja: Consultancy, Honoraria; Adicet: Research Funding; Allogene: Research Funding; Mustang Bio: Consultancy, Honoraria; 2Seventy Bio: Research Funding; Fate Therapeutics: Research Funding; NA: Patents & Royalties: cGVHD patent holder for Ibrutinib as cGVHD therapy but no compensation; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: rights to royalties from Fred Hutch for patents licensed to Juno, Research Funding; Legend Biotech: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Miltenyi: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Bioline Rx: Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Janssen: Consultancy, Honoraria, Other: Travel support. Ritz:TScan Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Garuda Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Clade Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Research Funding; Equillium: Research Funding; Oncternal: Research Funding; LifeVault Bio: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Smart Immune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Avrobio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Akron Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Obinutuzumab is a 2nd generation monoclonal antibody therapy directed against CD20. It is being tested as a B cell depletion strategy to prevent chronic GVHD.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal